|
Where are you now? Intelligence Services > Endotoxin > Endotoxin
Endotoxin
The purified form of endotoxin is called
"Lipopolysaccharide" or "LPS". Endotoxin is toxic and should
be handled with care.
Endotoxin is heat resistant and stable in most environmental
conditions. For example, it can only be destroyed at high temperatures (185°C for 3 hours or 250°C for 30 min.); by aggressive
chemicals such as NaOH or H2O2; or removed
by expensive chromatographic processes. Consequently, it can be
a major problem source in contamination control.
Endotoxin control is a critical quality requirement in the
manufacture of parenterals, biologicals, medical devices, and in the operation
of dialysis centers, blood donor centers, and in other areas specifically
regulated for endotoxin content and the prevention of endotoxin contamination.
For several decades the determination of endotoxin levels were
regulated by national & international agencies. In 2001, the International
Conference on Harmonization (ICH) issued a guideline
for endotoxin testing which has been adopted by the major pharmacopoeias, such
as the USP (United States Pharmacopoeia), EP (European Pharmacopoeia) BP
(British Pharmacopoeia), and JP (Japanese Pharmacopoeia). The techniques that
were accepted include:
- Gel-clot limit test
- Gel-clot assay
- Endpoint Turbidimetric
- Kinetic Turbidimetric
- Endpoint Chromogenic
- Kinetic Chromogenic
From a legal view-point, Gel-clot tests are defined as the
standard reference method, except in cases of approved applications such as
NDAs, ANDAs, etc., where other techniques can be specified as being legally
valid.
Endotoxin standards set the foundation for any of the testing
methodologies. The current reference standard (RSE) has been developed through
international collaboration, and may be purchased from the USP as EC-6.
Secondary standards such as Control Standard Endotoxin (CSE) may
be established and used in determinations provided that the standardization
procedure relative to RSE is in compliance with the regulation.
Endotoxin results are expressed as EU/unit or IU/unit. These
will be; EU/ml, or EU/ng. IU/ml, or IU/ng; 1 EU = 1 IU.
Special Definitions, Meanings, and Utilities
- EndotographTM – Definition:
Endotographs are transformed Gaussian functions of LAL-endotoxin kinetic
reaction data,
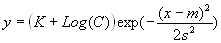
where,
-
C is the concentration,
- m is the x-coordinate of the inflection points (averaged over the
original replicate curves),
-
s represents the standard deviation of the x-coordinates from the
original replicate curves,
-
K =1 - INT(log(CMIN)-1), if INT(log(CMIN))
= log(CMIN) and CMIN < 1, or
K =1 - INT(log(CMIN)), where CMIN
is the least concentration value during the test.
The relationship between the maximum of the transformed
Gaussian-function (yMAX) and the concentration (C)
will be:
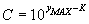 . .
-
EndotographTM – Meaning: Endotograph means acquiring
valuable new information on the specificity of the LAL-endotoxin kinetic
reaction curves. For example, it can differentiate between signal drift and the
reaction signals of LAL-endotoxin. This is of particular importance for the
lower concentrations of endotoxin standards and samples where the on-set
reaction times due to signal drift will give false results.
-
EndotographTM – Utility: Endotograph can be
generated from LAL-endotoxin kinetic reaction data by the PyroSolution program.
If OOS (out-of-specification) or OOT (out-of-trend) occurs during measurement,
Endotographs can then be utilized to review specificity characteristics of the
run, and will assist in determination if specificity requirements have been met.
-
EndotographTM – Competition: Can you generate
Endotographs from competitor products? NO! Should I invest in purchasing
PyroSolution? YES! Consider the following benefits: evaluate & improve
specificity, reduce OOS & OOT. An equally important feature of PyroSolution
is its full compliance with 21 CFR Part 11; while remaining less
expensive than other products offered for LAL-endotoxin measurements.
-
EndotogramTM – Definition: Endotograms
are transformed derivatives of the LAL-endotoxin kinetic reaction data, such
that the relationship between the area under the curve and endotoxin
concentration is defined by: C = 10A-K, where,
- C is the endotoxin concentration,
- A is the area under the curve,
- CMIN is the least Concentration, and
- K = 1 – INT(log(CMIN) – 1), if INT(log(CMIN) = log (CMIN) and CMIN
< 1, or
- K = 1 – INT(log(CMIN)
-
EndotogramTM – Meaning: Endotogram may be considered
as the "raw" form of the Endotograph. It complements Endotograph
information, and assists in evaluating the "quality" of the
LAL-endotoxin reaction measurement. For example, noisy peaks can indicate an
optical density gradient across the wells that may be due to temperature
turbulence, or poor homogeneity of the solution.
-
EndotogramTM – Utility : Endotograms can be
generated from LAL-endotoxin kinetic reaction data by the PyroSolution program.
They can provide interpretive support for method development during OOS &
OOT investigations.
-
EndotogramTM – Competition: Can you generate
Endotograms from competitive products? NO! Should I invest in purchasing
PyroSolution? YES! Consider the following benefits: evaluate & improve
reaction quality, reduce OOS & OOT. An equally important feature of
PyroSolution is its full compliance with 21 CFR Part 11; while remaining
less expensive than other products offered for LAL-endotoxin measurements.
-
Robust Kinetics – Definition: Robust kinetic is a
kinetic methodology, that includes multiple point – up to 35 – optical
density data for a specific LAL-endotoxin kinetic reaction time in each
microplate well, which is then coupled with an averaging algorithm to reduce the
optical density gradient within each well.
-
Robust Kinetics – Meaning: Robust kinetics means a far greater
reliability and improved quality for kinetic turbidimetric measurements.
-
Robust Kinetics – Utility: Robust kinetics can be defined in
the PyroSolution program, and is recommended during kinetic turbidimetric
measurements. You can extend the capability of turbidimetric kinetic tests by
improving reproducibility, and by reducing OOS & OOT.
-
Robust Kinetics – Competition: Can you define Robust Kinetics
in competitor products? NO! Should I invest in purchasing PyroSolution? YES!
Consider the following benefits: improved reliability of kinetic turbidimetric
tests, reduce OOS & OOT. An equally important feature of PyroSolution is its
full compliance with 21 CFR Part 11; while remaining less expensive than
other products offered for LAL-endotoxin measurements.
-
Endotoxin Measurement Uncertainty (EMU) – Definition:
EMU is defined as the data associated with an endotoxin result, that is a
contributor to the dispersion of the measurement that characterizes the
determination process.
-
Endotoxin Measurement Uncertainty (EMU) – Meaning: EMU provides
meaningful information on the inherent error load of the analytical process. For
example, it helps to evaluate the quality constraint of a measurement and
highlight, which parameter(s) must be improved to significantly reduce
uncertainty while improving accuracy.
-
Endotoxin Measurement Uncertainty (EMU) – Utility: EMU can be
generated from PyroSolution. In addition to evaluating existing methods &
results, it can also greatly improve the method development process, and
investigations relative to OOS & OOT.
-
Endotoxin Measurement Uncertainty (EMU) – Competition: Can you
generate Endotoxin Measurement Uncertainty from competitive products? NO!
- Pyroburden – Definition: Pyroburden is the endotoxin
load for a process Pi or Processes
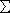 Pi relative
to time and space. Pyroburden exists if and only if quantitative endotoxin data
exists. Pyroburden is expressed by its characteristic parameters jointly
referred to as the Pyroburden Characteristic Set (PCS). PCS includes: Endotoxin
Area Under the Curve (EAUC), Maximum Endotoxin Concentration (MEC), Number of
Endotoxin Peaks (NEP), Minimum Endotoxin Concentration (NEC), Time-to-Maximum
(TOM), Time-to-Minimum (TON). Pi relative
to time and space. Pyroburden exists if and only if quantitative endotoxin data
exists. Pyroburden is expressed by its characteristic parameters jointly
referred to as the Pyroburden Characteristic Set (PCS). PCS includes: Endotoxin
Area Under the Curve (EAUC), Maximum Endotoxin Concentration (MEC), Number of
Endotoxin Peaks (NEP), Minimum Endotoxin Concentration (NEC), Time-to-Maximum
(TOM), Time-to-Minimum (TON).
-
Pyroburden – Internal – Definition: Pyroburden-Internal is
the Pyroburden of materials and equipment used.
-
Pyroburden – External – Definition: Pyroburden-External is
the Pyroburden of the environment and people.
-
Pyroburden – Meaning: Pyroburden may be considered as the
endotoxin contamination risk factor associated with a process. Endotoxin load
patterns – based on the Pyroburden characteristics parameters - can be
established for each process, and during pyroburden monitoring, statistically
significant deviations can be used for predictions.
-
Pyroburden – Utility: Pyroburden can be generated from the
PyroSolution program. Two modules – Sample Tracking & EQU – of
PyroSolution provide the basis for pyroburden capability. In addition to
evaluating the endotoxin risk that may be inherent during processing, cleaning
validation can also be supported by PCS. This technology may also be applied for
bioburden analysis.
-
Pyroburden – Competition: Can you obtain pyroburden in
competitor products? NO! Should I invest in purchasing PyroSolution? YES!
Consider the following benefits: improved understanding of endotoxin
contamination, control, management, and intervention using knowledge in hand.
Reduce OOS & OOT, and improve compliance. An equally important feature of
PyroSolution is its full compliance with 21 CFR Part 11; while remaining
less expensive than other products offered for LAL-endotoxin measurements.
-
PyroSolution – is a 21 CFR Part 11 compliant software for
the Endotoxin Information Management System. The program includes a control
& measurement module for various microplate readers, such as BioTek’s EL808,
BioTek’s PowerWave, BioTek’s CERES900, BioTek’s EL340, BioWhittaker’s
KQCL, Anthos’ HTIII, Labsystems’ Twinreader, AsysHitech’s DigiScan.
PyroSolution is able to control readers in a parallel mode; for example, 2 x
BioTek EL808 & 2 x KQCL readers – 4 at the same time – can be used in
parallel operation with a single SQL database. Integrated Sample Tracking and
Endotoxin Decision Management Support extends the capability of the programs
into a fully-fledged Endotoxin Information Management System. PyroSolution
utilizes a sophisticated signal processing technology to improve accuracy,
reproducibility, sensitivity, specificity, and robustness of raw data. Should I
consider buying PyroSolution? YES! In addition to the benefits that you have
already read; there are many, many more! But don’t just take our word, order
a demo version for trial and see for yourself.
-
PyroGel – is a 21 CFR Part 11 compliant software for
gel-clot determinations of Endotoxin. The program utilizes ICH protocols for the
measurements. A sophisticated graphical wizard is used for set up, load, &
calculations, while advanced reporting & modeling capabilities extend its
utility from routine endotoxin QC to endotoxin QC/QA management. The software
controls and monitors the set temperature at 8 different points of the PyroTherm
temperature block thereby improving reliability, while providing prompt and full
documentation. Included also is single process temperature programming for
depyrogenation, cooling, and gel-clot measurements. Data can also be integrated
with the quantitative results obtained in PyroSolution. Should I consider buying
PyroGel? YES! In addition to the benefits that you have already read; there are
many, many more! But don’t just take our word, order
a demo version for trial and see for yourself.
-
PyroDoQ – is a Design Qualification (DQ) & method
development software solution product for LAL-endotoxin measurement. DQ may be
the most critical, but least understood component of the DQ-IQ-OQ-PQ process. DQ
is tightly integrated to PQ! You cannot get more performance specification, or
performance quality, or performance stability out of a system, than has been
built into the system! What specification & qualities do I need for my
measurements? PyroDoQ provides accurate information of the specification &
quality characteristics required for a particular application. Should I consider
buying PyroDoQ? YES! In addition to the benefits that you have already read;
there are many, many more! PyroDoQ reduces the risk of getting the wrong system.
You can also synchronize DQ with PQ!
Order
a demo version for trial and see for yourself.
- PyroSuite – is a
collection of software solution products developed to cover all of the
requirements for endotoxin testing, endotoxin control, endotoxin information
management & endotoxin executive management. It includes the PyroSolution,
PyroGel, and PyroDoQ software packages with appropriate database & EQU
integration. Is PyroSuite more than PyroSolution, PyroGel, and PyroDoQ in a box?
YES! PyroSuite offers added database integration and integrated EQU models that
will optimize method development, and endotoxin control, to develop an endotoxin
knowledge base; and for the execution of effective and efficient prevention of
endotoxin contamination while achieving maximum economic benefits.
- EQU* – is an SQL query & modeling Application
Integration System. It can connect to multiple databases and extract information
for an integrated model. It can also be considered as a knowledge engineering
tool; for example, to create a new database for extracted information for use in
a recursive mode. EQU* also uses a natural language interface with embedded
parameters and dynamic sentence creation for SQL-queries. EQUANT and EQUAL are
derivatives of EQU*; EQUANT is a real-time extension of EQU, whereas, EQUAL is a
library of EQU applications.
Endotoxin Safety & Hazard
Measures
- It may be fatal if absorbed or introduced through skin,
- In the case of contact, immediately remove contaminated clothing and
flush the skin area with plenty of water for at least 15 minutes.
- Cover open cuts before use.
- Harmful if inhaled,
- Avoid breathing dust,
- If inhaled, remove to fresh air.
- If injected,
- Seek immediate medical help.
- If breathing has stopped,
- Give mouth-to-mouth resuscitation,
- If breathing is difficult, give oxygen,
- Seek immediate medical help.
When working with endotoxin, the following basic safety
precautions should be taken:
- Use dust masks,
- Protective rubber gloves are recommended,
- Goggles are recommended for eye protection,
- Wash thoroughly after handling.
Accidental Spillage and Disposal
- If spilled or released,
- Sweep up solid matter & incinerate
- Mop remainder and flush down drain with plenty of water.
- In case of disposal, incineration is recommended.
|
|
    

|
|
